Enzymes biological catalysts worksheet answer key – The Enzymes: Biological Catalysts Worksheet Answer Key provides a comprehensive overview of the fundamental concepts and principles governing enzymes, their role in biological reactions, and their regulation. This resource is designed to enhance understanding and reinforce key takeaways from the accompanying worksheet, empowering students with a deeper comprehension of enzyme function and their significance in biological processes.
Throughout this guide, we will explore the intricate mechanisms of enzyme-substrate interactions, delve into the kinetics of enzyme activity, and examine the various types of enzyme inhibition. Furthermore, we will investigate the critical role of enzyme regulation in maintaining cellular homeostasis and discuss practical applications of enzyme inhibition in medicine and industry.
1. Enzymes
Biological Catalysts
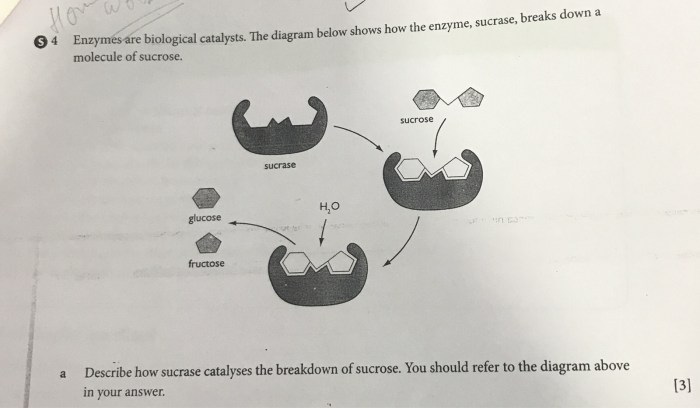
Enzymes are biological molecules that act as catalysts in biochemical reactions. They increase the rate of reactions without being consumed or permanently altered in the process. Enzymes are essential for life as they enable the efficient and specific conversion of substrates into products in living organisms.Enzymes
are highly specific, meaning they only catalyze a particular reaction or a narrow range of related reactions. They are composed of amino acids and can be classified into different types based on their structure and catalytic mechanisms. Some common types of enzymes include:
- Oxidoreductases: Transfer electrons between molecules.
- Transferases: Transfer functional groups between molecules.
- Hydrolases: Break down molecules by adding water.
- Lyases: Break down molecules by removing a group without adding water.
- Isomerases: Rearrange atoms within a molecule.
- Ligases: Join two molecules together.
The activity of enzymes is influenced by various factors, including temperature, pH, substrate concentration, and the presence of inhibitors or activators.
2. Enzyme-Substrate Interactions
Enzymes interact with their substrates through specific binding sites called active sites. The active site is a region on the enzyme that is complementary in shape and charge to the substrate. There are two main models that describe enzyme-substrate interactions:
- Lock-and-Key Model:This model suggests that the enzyme and substrate have a rigid, complementary fit, like a lock and key. The substrate binds to the active site with high specificity.
- Induced-Fit Model:This model proposes that the enzyme undergoes a conformational change upon substrate binding. The active site adjusts its shape to accommodate the substrate, leading to a more stable enzyme-substrate complex.
The active site of an enzyme contains specific amino acid residues that participate in substrate binding and catalysis. These residues create an environment that facilitates the chemical reaction and lowers the activation energy required for the reaction to occur.
3. Enzyme Kinetics: Enzymes Biological Catalysts Worksheet Answer Key
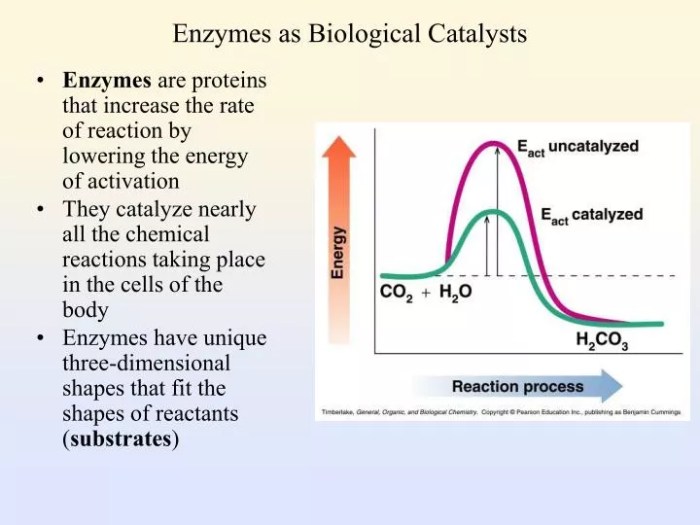
Enzyme kinetics is the study of the rate of enzyme-catalyzed reactions. The Michaelis-Menten equation is a mathematical model that describes the relationship between the reaction rate, substrate concentration, and enzyme concentration. The equation is:$$v = \fracV_max [S]K_m + [S]$$where:
- $v$ is the reaction rate.
- $V_max$ is the maximum reaction rate.
- $[S]$ is the substrate concentration.
- $K_m$ is the Michaelis constant, which represents the substrate concentration at which the reaction rate is half of $V_max$.
Factors that affect enzyme kinetics include temperature, pH, enzyme concentration, substrate concentration, and the presence of inhibitors or activators.
4. Enzyme Inhibition

Enzyme inhibition is the process by which the activity of an enzyme is decreased or blocked. Inhibitors are molecules that bind to enzymes and interfere with their function. There are three main types of enzyme inhibition:
- Competitive Inhibition:The inhibitor competes with the substrate for binding to the active site. The inhibitor resembles the substrate in structure and binds to the active site, preventing the substrate from binding.
- Non-Competitive Inhibition:The inhibitor binds to the enzyme at a site other than the active site. This binding causes a conformational change in the enzyme that reduces its catalytic activity.
- Uncompetitive Inhibition:The inhibitor binds to the enzyme-substrate complex, causing a conformational change that reduces the catalytic activity of the enzyme.
Enzyme inhibition has applications in medicine and industry. For example, drugs can be designed to inhibit specific enzymes involved in disease processes, and inhibitors can be used to control enzyme activity in industrial processes.
5. Enzyme Regulation
Enzyme regulation is essential for maintaining cellular homeostasis and coordinating metabolic pathways. There are various mechanisms by which enzymes are regulated, including:
- Allosteric Regulation:Allosteric enzymes have multiple binding sites. The binding of a regulatory molecule (effector) to an allosteric site can alter the enzyme’s catalytic activity.
- Feedback Inhibition:The end product of a metabolic pathway can bind to an enzyme earlier in the pathway and inhibit its activity. This negative feedback mechanism helps prevent the overproduction of the end product.
- Covalent Modification:Enzymes can be modified by covalent attachment of molecules such as phosphate groups or acetyl groups. These modifications can alter the enzyme’s activity or stability.
Enzyme regulation is crucial for maintaining cellular balance, responding to environmental cues, and coordinating complex biological processes.
Questions and Answers
What is the primary function of enzymes?
Enzymes act as biological catalysts, facilitating and accelerating specific chemical reactions within living organisms.
How do enzymes achieve their catalytic activity?
Enzymes possess active sites that bind to specific substrates, providing a favorable environment for the reaction to occur, thereby lowering the activation energy required for the reaction.
What are the different types of enzyme inhibition?
Enzyme inhibition can be classified into three main types: competitive, non-competitive, and uncompetitive inhibition, each with distinct mechanisms of action.