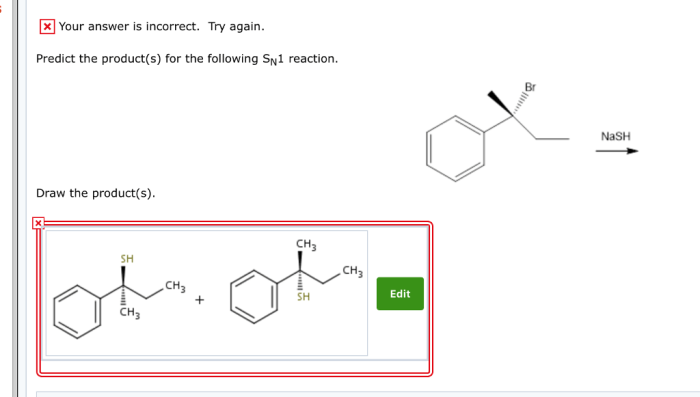
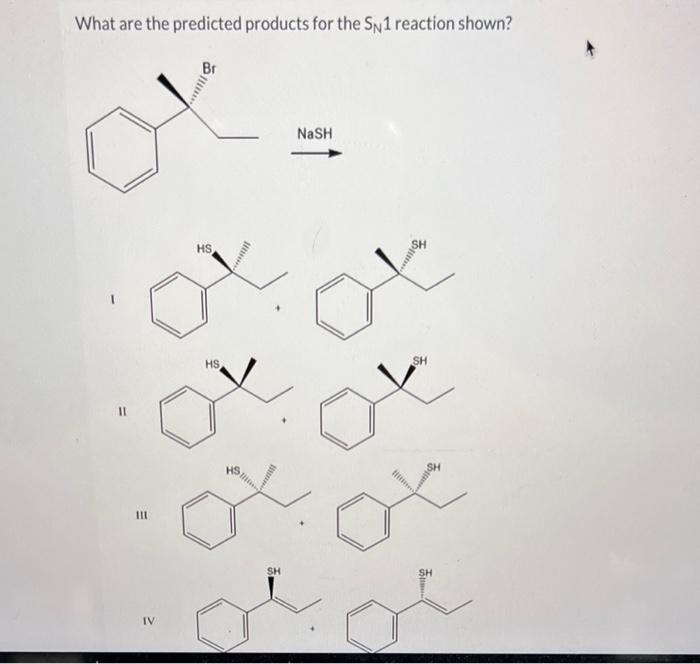
What are the predicted products for the SN1 reaction shown? This question is at the heart of understanding nucleophilic substitution reactions, a fundamental concept in organic chemistry. SN1 reactions proceed through a two-step mechanism involving the formation of a carbocation intermediate.
The nature of the substrate, nucleophile, and solvent can significantly influence the rate and selectivity of the reaction, ultimately determining the predicted products.
This article delves into the intricacies of SN1 reactions, exploring the factors that govern their behavior and showcasing their applications in organic synthesis. By unraveling the mechanisms and principles underlying these reactions, we gain a deeper appreciation for the complexities of chemical reactivity and the power of predicting reaction outcomes.
SN1 Reactions
SN1 reactions are a type of nucleophilic substitution reaction in which the rate-determining step involves the ionization of the substrate to form a carbocation, followed by a fast reaction of the carbocation with the nucleophile.
Substrate Structure and Reactivity
The structure of the substrate has a significant influence on the rate and selectivity of the SN1 reaction. Substrates that are able to form stable carbocations undergo SN1 reactions more readily. For example, tertiary alkyl halides undergo SN1 reactions much faster than primary alkyl halides.
Nucleophile Strength and Selectivity
The strength of the nucleophile also affects the rate and selectivity of the SN1 reaction. Strong nucleophiles react faster with carbocations than weak nucleophiles. However, strong nucleophiles can also lead to more substitution products, as they can react with the carbocation before it has a chance to rearrange.
Solvent Effects
The polarity of the solvent can also affect the rate and selectivity of the SN1 reaction. Polar solvents stabilize carbocations, which makes them more likely to form and react with nucleophiles. As a result, SN1 reactions are more likely to occur in polar solvents.
Stereochemistry
SN1 reactions typically proceed with inversion of configuration at the carbon atom that is being substituted. This is because the carbocation intermediate is planar, and the nucleophile can attack from either side.
Applications of SN1 Reactions, What are the predicted products for the sn1 reaction shown
SN1 reactions are used in a variety of synthetic applications. For example, SN1 reactions can be used to prepare alkenes, alcohols, and ethers. SN1 reactions are also used in the synthesis of natural products and pharmaceuticals.
Detailed FAQs: What Are The Predicted Products For The Sn1 Reaction Shown
What is the key intermediate in an SN1 reaction?
A carbocation
How does nucleophile strength affect the rate of an SN1 reaction?
Nucleophile strength has no effect on the rate of an SN1 reaction.
What is the stereochemical outcome of an SN1 reaction?
Inversion of configuration