N butyl acetate ir spectrum – Embark on a captivating journey into the realm of infrared spectroscopy, where we unravel the secrets of n-butyl acetate’s molecular structure through its unique IR spectrum. Delve into the intricacies of this essential analytical tool, exploring its principles, applications, and significance in understanding the chemical world.
n-Butyl acetate, a versatile solvent with diverse industrial uses, reveals its molecular fingerprint through its IR spectrum. Each absorption band, a testament to specific functional groups, provides a window into the compound’s chemical composition. This guide illuminates the relationship between molecular structure and IR spectral features, empowering you to decipher the language of molecules.
Infrared Spectroscopy Basics
Infrared spectroscopy is a powerful analytical technique that provides information about the molecular structure and composition of a sample. It is based on the principle that molecules absorb infrared radiation at specific frequencies that correspond to the vibrational modes of their atoms and functional groups.
There are two main types of infrared spectra: absorption spectra and emission spectra. Absorption spectra are recorded by passing infrared radiation through a sample and measuring the amount of radiation that is absorbed at each frequency. Emission spectra are recorded by exciting a sample with infrared radiation and measuring the amount of radiation that is emitted at each frequency.
Infrared spectroscopy is used in a wide variety of applications, including the identification of organic and inorganic compounds, the analysis of polymers, and the study of surface chemistry.
Instrumentation
The instrumentation used in infrared spectroscopy consists of a light source, a sample holder, a detector, and a recorder. The light source is typically a heated filament or a globar. The sample holder is used to hold the sample in the path of the infrared radiation.
The detector is used to measure the amount of infrared radiation that is absorbed or emitted by the sample. The recorder is used to record the spectrum.
n-Butyl Acetate
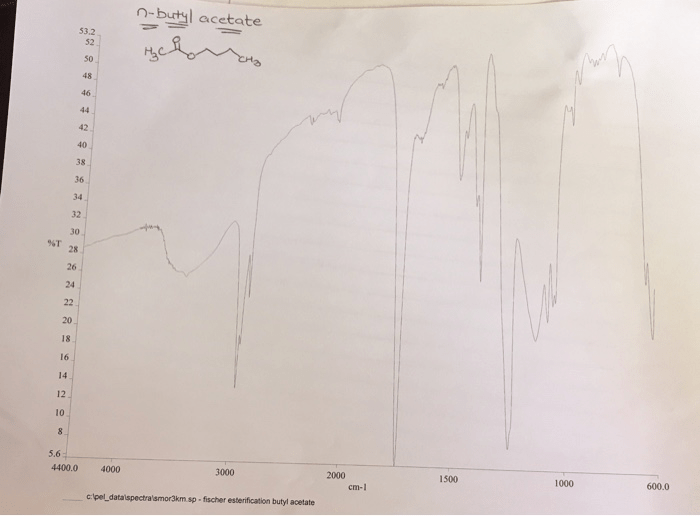
n-Butyl acetate is an organic compound with the formula CH3COOCH2CH2CH2CH3. It is a colorless liquid with a fruity odor. n-Butyl acetate is a common solvent and is used in the manufacture of paints, lacquers, and adhesives.
Chemical Structure and Properties
n-Butyl acetate is an ester, which means it is formed by the reaction of a carboxylic acid with an alcohol. In this case, the carboxylic acid is acetic acid and the alcohol is n-butanol. The chemical structure of n-butyl acetate is shown below:“`CH3COOCH2CH2CH2CH3“`n-Butyl acetate is a nonpolar molecule.
It is insoluble in water but soluble in organic solvents such as ethanol and ether. n-Butyl acetate has a boiling point of 126 °C and a melting point of
78 °C.
Characteristic Functional Groups
The characteristic functional group in n-butyl acetate is the ester group (-COO-). The ester group is a polar functional group, which means it has a dipole moment. The dipole moment of the ester group is due to the difference in electronegativity between the oxygen and carbon atoms.
Physical and Chemical Properties
n-Butyl acetate is a flammable liquid. It has a flash point of 24 °C and an autoignition temperature of 420 °C. n-Butyl acetate is also a reactive compound. It can undergo hydrolysis, oxidation, and reduction reactions.
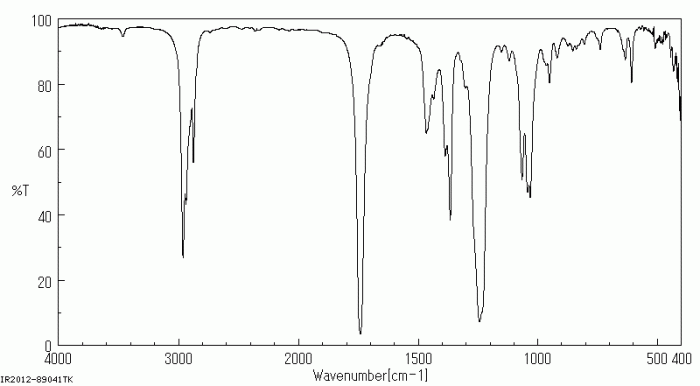
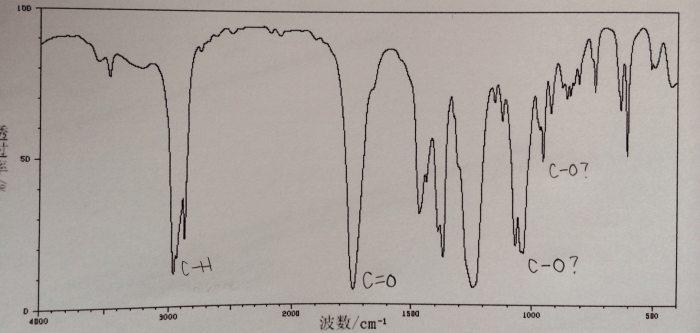
IR Spectrum of n-Butyl Acetate
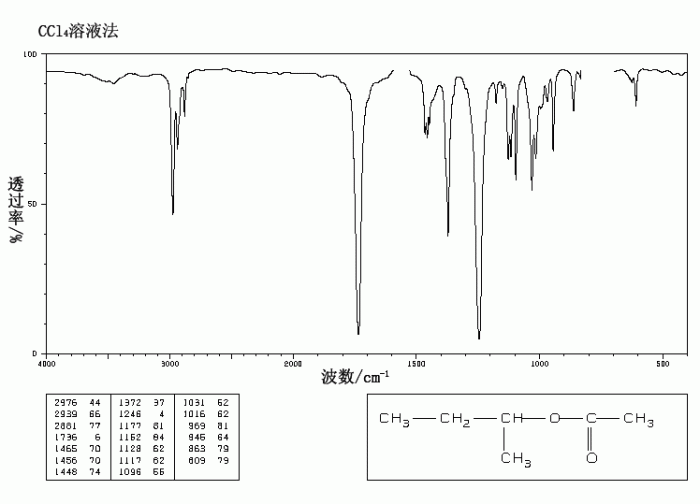
The IR spectrum of n-butyl acetate exhibits several characteristic absorption bands that provide valuable information about the functional groups present in the molecule.
N butyl acetate IR spectrum is a useful tool for identifying this organic compound. In the context of logistic growth, the carrying capacity can be calculated using a differential equation similar to the one used in logistic growth ap calc bc . Returning to the n butyl acetate IR spectrum, the characteristic peaks can be assigned to specific functional groups, providing insights into its molecular structure.
Identification of Key Absorption Bands
- C-H Stretching (2960-2870 cm-1): This band arises from the stretching vibrations of the C-H bonds in the alkyl groups.
- C=O Stretching (1735 cm-1): This strong band is characteristic of the carbonyl group (C=O) in the ester functional group.
- C-O Stretching (1240 cm-1): This band is attributed to the stretching vibrations of the C-O bond in the ester group.
- O-C-C Stretching (1160 cm-1): This band arises from the stretching vibrations of the O-C-C bond in the ester group.
Relationship between Molecular Structure and IR Spectrum
The IR spectrum of n-butyl acetate reflects the molecular structure of the compound. The presence of the strong C=O stretching band at 1735 cm -1confirms the presence of the ester functional group. The C-O and O-C-C stretching bands further support this assignment.
The alkyl groups give rise to the C-H stretching bands in the 2960-2870 cm -1region.
Applications of IR Spectroscopy in n-Butyl Acetate Analysis: N Butyl Acetate Ir Spectrum
Infrared (IR) spectroscopy is a powerful analytical technique used to identify and quantify functional groups in organic compounds. It is a non-destructive technique that can be used to analyze solid, liquid, or gas samples.
IR spectroscopy has a wide range of applications in the analysis of n-butyl acetate. It can be used to:
- Identify the functional groups present in n-butyl acetate
- Quantify the amount of n-butyl acetate in a sample
- Determine the purity of n-butyl acetate
- Monitor the reaction progress of n-butyl acetate
- Identify the impurities present in n-butyl acetate
IR spectroscopy is a versatile technique that can provide a wealth of information about n-butyl acetate. It is a relatively simple and inexpensive technique to use, making it a valuable tool for a wide range of applications.
Advantages of Using IR Spectroscopy for n-Butyl Acetate Analysis
There are several advantages to using IR spectroscopy for n-butyl acetate analysis. These advantages include:
- IR spectroscopy is a non-destructive technique, meaning that the sample does not need to be destroyed in order to be analyzed.
- IR spectroscopy is a relatively simple and inexpensive technique to use.
- IR spectroscopy can be used to identify and quantify functional groups in organic compounds.
- IR spectroscopy can be used to analyze solid, liquid, or gas samples.
Limitations of Using IR Spectroscopy for n-Butyl Acetate Analysis, N butyl acetate ir spectrum
There are also some limitations to using IR spectroscopy for n-butyl acetate analysis. These limitations include:
- IR spectroscopy cannot be used to determine the structure of a molecule.
- IR spectroscopy can be affected by the presence of water in the sample.
- IR spectroscopy can be difficult to interpret if the sample is complex.
Comparison with Other Techniques
Infrared (IR) spectroscopy is one of several techniques used to analyze n-butyl acetate. Other common techniques include gas chromatography (GC) and mass spectrometry (MS).
Each technique has its advantages and disadvantages, and the choice of which technique to use depends on the specific needs of the analysis.
Gas Chromatography
Gas chromatography (GC) is a technique that separates and analyzes compounds based on their volatility and affinity for a stationary phase. GC is often used to analyze volatile compounds, such as n-butyl acetate, in complex mixtures.
Advantages of GC:
- High sensitivity
- Can be used to analyze complex mixtures
- Can be used to identify and quantify compounds
Disadvantages of GC:
- Can be time-consuming
- Requires specialized equipment
- Can be destructive to the sample
Mass Spectrometry
Mass spectrometry (MS) is a technique that identifies and quantifies compounds based on their mass-to-charge ratio. MS is often used to identify unknown compounds or to confirm the identity of known compounds.
Advantages of MS:
- High specificity
- Can be used to identify unknown compounds
- Can be used to quantify compounds
Disadvantages of MS:
- Can be expensive
- Requires specialized equipment
- Can be destructive to the sample
When to Use Each Technique
The choice of which technique to use for n-butyl acetate analysis depends on the specific needs of the analysis. If the goal is to identify an unknown compound, MS is the best choice. If the goal is to quantify n-butyl acetate in a complex mixture, GC is the best choice.
If the goal is to obtain structural information about n-butyl acetate, IR spectroscopy is the best choice.
Commonly Asked Questions
What is the principle behind infrared spectroscopy?
Infrared spectroscopy utilizes the absorption of infrared radiation by molecules, causing vibrations within their chemical bonds. These vibrations correspond to specific functional groups, providing insights into molecular structure.
How can IR spectroscopy be used to identify n-butyl acetate?
The IR spectrum of n-butyl acetate exhibits characteristic absorption bands corresponding to its functional groups, such as the C-O stretching vibration of the ester group. By matching these bands to reference spectra, n-butyl acetate can be readily identified.
What are the limitations of IR spectroscopy for n-butyl acetate analysis?
While IR spectroscopy provides valuable information about functional groups, it may not always distinguish between isomers or provide detailed structural information. Additionally, IR spectra can be affected by sample preparation and environmental factors.